The breakdown of chlorofluorocarbons (CFCs) in the stratosphere has been implicated in the destruction of Earth's protective ozone layer. Consequently, scientists have undertaken studies to better understand the structure and behavior of highly reactive, but short-lived, free radicals produced during the breakdown process. The molecules, which contain either fluorine or chlorine, are an important source of atmospheric halogen atoms. Elucidating their 3D structure and dynamical behavior will help scientists better understand atmospheric chemistry as well as their fundamental molecular properties.
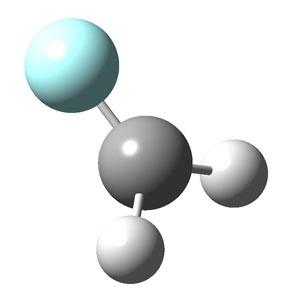
Using slit-jet supersonic expansion and high-resolution infrared spectroscopy, JILA researchers have determined key vibration modes of both the chloromethyl and fluoromethyl radicals. The latter is shown below (the fluorine atom is teal, the carbon atom dark grey, and two hydrogen atoms light grey). Erin Whitney, who earned her Ph. D. in January and is now at the National Renewable Energy Laboratory, Research Associate Feng Dong, and Fellow David Nesbitt were also able to resolve exquisite features in the molecular spectra that permitted them to deduce the structures of the two radicals.
Whitney and her colleagues were able to detect two stretching patterns for the carbon-hydrogen bonds in fluoromethyl radical. In one mode, the two hydrogens move toward and away from the carbon at the same time (symmetric); in the other, one hydrogen moves in as the other moves out (antisymmetric). This observation, in conjunction with a theoretical analysis, allowed the researchers to figure out that the four atoms of this molecule do not lie in the same plane; rather, the fluorine atom bends up at an angle of 29°. They also deduced that CH stretch vibrations make the molecule's electrical charge "slosh" among the fluorine, carbon, and hydrogen atoms in a surprisingly counterintuitive way. Usually the carbon-hydrogen bond forms a dipole, with the bond's negatively charged electron wave function hanging out nearer the carbon atom. This is also true for CH2F radical, but the presence of the "electron hungry" F atom makes the charges flow in a direction that tries to counteract this dipole. This has major consequences for the relative intensities of different vibrations in the observed spectrum.
The researchers found that charge sloshing even more strongly impacts chloromethyl radical. Charge sloshing virtually eliminates the antisymmetric vibration mode for the carbon-hydrogen bonds and flattens chloromethyl's 3D shape. Even though chlorine is larger than fluorine, it bends up only about 11° and may spend some time in the same plane as the other atoms. Although the researchers were quite surprised by the differences between chloromethyl and fluoromethyl radicals, their theoretical calculations strongly supported their experimental observations. - Julie Phillips